Carbon can combine with itself and with many other elements to form a great diversity of compounds. Carbon is a nonmetal in group 14 in the periodic table. Carbon bonding, They need four more electrons to fill the outer shell to make this element stable by forming covalent bod (share with nonmental and mental) by share four of the electrons. Carbon often forming with Hydrogen. Carbon can form single, double, or even triple bonds with other carbon atoms. In a single bond, two carbon atoms share one pair of electron. In a double bond, they share two pairs of electrons, and in a triple bond, they share three pairs of electrons. Because of carbon’s ability to form so many covalent bonds, it often forms polymers. A polymer is a large molecule that consists of many smaller molecules joined together by covalent bonds. The smaller molecules are called monomers. (The prefix mono means “one,” and the prefix poly means “many.”) . Pure carbon can exist in different forms, depending on how its atoms are arranged. When they form, they will form different structure and it will have different properties, including diamond, graphite, and fullerenes. Carbon is a nonmetal with four valence electrons. Each carbon atom forms four covalent bonds. We call this element is “ Element of Life”.
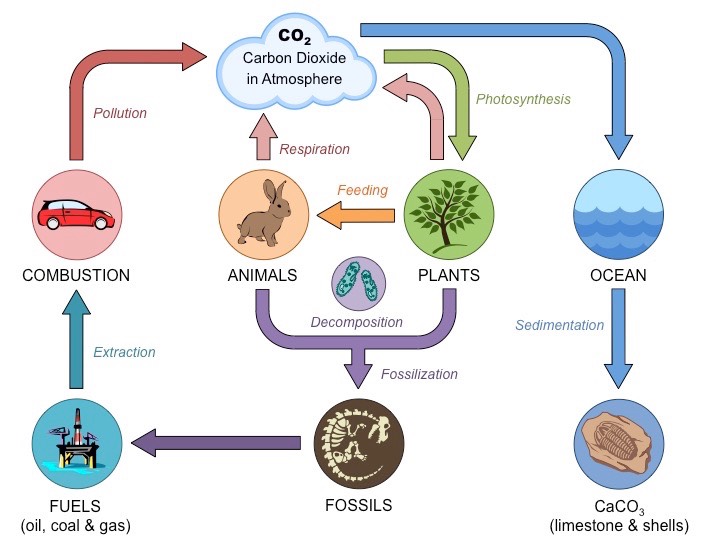
The Carbon cycle
From Bioninja